06 Oct The usefulness of metals in structural and other applications depends on their physical and chemicalproperties. Although iron is the most common metal used in manufacturing, it must be pro
Virtual Lab: Activity Series
The usefulness of metals in structural and other applications depends on their physical and chemical
properties. Although iron is the most common metal used in manufacturing, it must be protected against
corrosion because rusts easily. Copper is used in electrical wiring because it conducts electricity extremely well and resists corrosion better than many metals. Gold is a highly valuable jewelry metal because it is essentially unreactive. How can we determine the relative reactivity of different metals?
To determine the activity of metals you can compare the reactions of metals with different
metal ions. Consider equation 1 and 2 below:
2Al(s) + 3CuCl2(aq) –> 2AlCl3(aq) + 3Cu(s) (Equation 1)
Cu(s) + AlCl3(aq) –> No Reaction (Equation 2)
The reaction of aluminum with copper (II) chloride (Equation 1) is classified as a single replacement reaction – aluminum reacts with and “replaces” copper ions in copper (II) chloride. Single replacement reactions will occur spontaneously in one direction only (compare Equations 1 and 2). A more active metal always replaces the ion of a less active metal. In general, the activity of a metal may be defined as follows:
An active metal will react with a compound of a less active metal, which is converted to its “free element” form. The more active metal forms a new compound containing metal cations. Based on Equation 1, aluminum is more active than copper and therefore replaces the copper (this is called a single replacement reaction).
Objective:
-To explore the reactivity of metals
-To practice writing single replacement reactions
-To practice using the activity series chart in your reference table
Procedure:
1. Click on the link below or copy and paste it into your browser.
teachchemistry.org/classroom-resources/metals-in-aqueous-solutions-simulation
2. Click Activity 1.
3. Click CONTINUE at the bottom.
4. Pick Mg metal and click CONTINUE.
5. Click on each beaker to see the "molecular scale"
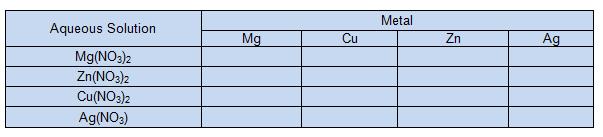
6. In data table 1, if a reaction occurred, write "reaction." If no reaction occurs write "no reaction".
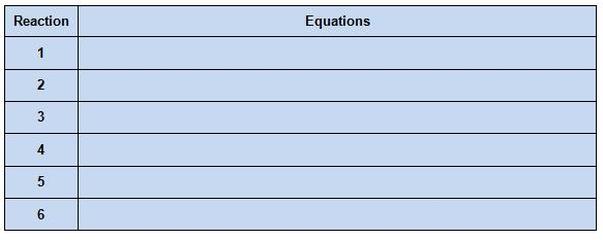
7. In table 2, write the balanced equations for the reactions that occured. (Hint: these are single replacement reactions)
8. Now repeat steps 1-7 for the next three metals (Cu, Zn and Ag).
TABLE 1
TABLE 2
Questions:
1. Which of the metals reacted with the most solutions?
2. Which of the metals reacted with the fewest solutions?
3. List the 4 Metals in order from the most reactive to the least reactive.
4. Refer to table J in your reference table. This is the activity series which lists the most reactive metals on top and the least reactive on the bottom. Compare your answer to question #3 with the activity series. Are your results in the same order? Why? (Click here for Table J)
5. If we added Pb to the list, which of the solutions would you expect it to react with?
Our website has a team of professional writers who can help you write any of your homework. They will write your papers from scratch. We also have a team of editors just to make sure all papers are of HIGH QUALITY & PLAGIARISM FREE. To make an Order you only need to click Ask A Question and we will direct you to our Order Page at WriteDemy. Then fill Our Order Form with all your assignment instructions. Select your deadline and pay for your paper. You will get it few hours before your set deadline.
Fill in all the assignment paper details that are required in the order form with the standard information being the page count, deadline, academic level and type of paper. It is advisable to have this information at hand so that you can quickly fill in the necessary information needed in the form for the essay writer to be immediately assigned to your writing project. Make payment for the custom essay order to enable us to assign a suitable writer to your order. Payments are made through Paypal on a secured billing page. Finally, sit back and relax.